The Risk-Reward Conundrum
Opportunities and challenges of bringing innovative therapies to market
The future for patients with genetic disorders or difficult to treat diseases has never been brighter, largely due to a wave of novel medicines based on genetic engineering,
innovative cell-based therapies and tissue-engineered products. These advanced therapies – called advanced therapy medicinal products (ATMPs) in Europe and the UK and cell and gene therapy products (CGTPs) in the US – are transforming the landscape of disease treatment and opening a new world of hope for patients who haven’t had it in the past.
” These advanced therapies are transforming the landscape of disease treatment and opening a new world of hope for patients who haven’t had it in the past.”
Yet, for all the excitement about the life-changing potential of these advancements, there is still the somber realization of significant known and unknown risks, many of which are unique to this product class.
For example, particularly challenging is the fact that nonclinical animal models are often of limited relevance or not available at all. Further, risks and uncertainties arise from the manufacturing process, the surgical procedures associated with the cell collection, or the method of administration.
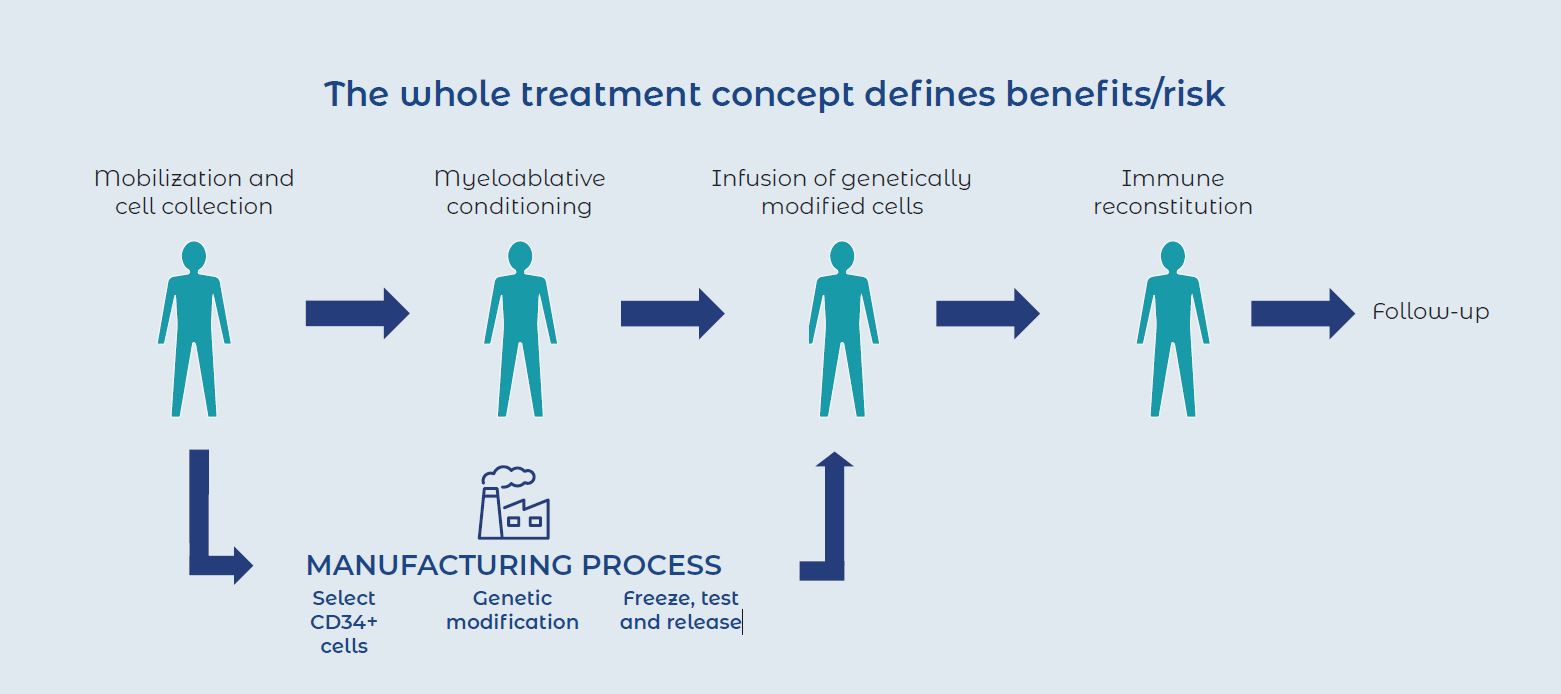
Knowing the risks also means controlling the risks, giving companies the opportunities to develop mitigation strategies that allow them to smoothly transition from the nonclinical to clinical stage.
Life-changing treatments. Unique challenges.
For hemophilia patients today looking forward to a lifetime of frequent prophylactic infusions of plasma-derived or recombinant factor VIII and monitoring, a one-dose gene therapy cure may be just around the corner. Genetic medicines for a range of diseases such as sickle cell anemia and several of the muscular dystrophies appear just in reach.
For diseases like Alzheimer’s disease, Parkinson’s disease, cancer, cardiomyopathies and more, ATMPs hold great potential for modifying the progression or the disability associated with these conditions. These examples illustrate how the introduction of ATMPs has been a game changer for the treatment of grave conditions that have no suitable therapies or very limited treatment options.
As transformative as these innovative therapies can be in terms of treatment potential, they also present unique challenges when it comes to risk. These include issues such as donor identification and traceability and ensuring cell quality during collection and transport. Their novel mechanisms of action as well as required concomitant procedures (such as surgery or myeloablative therapies) means that ATMPs may produce new risks to patients. There may also be risks to healthcare professionals and caregivers, such as virus shedding.

And then there are the financial considerations. It can take over a decade and $1 billion to bring a new therapy from discovery to the market.1 With those costs at stake, most organizations can’t afford costly missteps. They must consider strategies that will help them clear the obstacles that lie ahead and move them closer to bringing ground-breaking therapies to people in need.
Proactively reducing risk throughout the development cycle.
To leverage to full potential of the most promising candidates, organizations must proactively control the benefitrisk ratio from the beginning. With a flexible strategic plan centered on issues that can lead to failure at every point of the development lifecycle, companies can:
● Ease the financial burden,
● Reduce safety risks, and
● Mitigate issues associated with the regulatory process
At each point, risk should be assessed to ascertain whether development should continue— a “go/no-go” stage.
Even with a “go,” pivots may be necessary.
The focus, at a very high level, should be risk identification, evaluation and mitigation. We should be looking at not only the biological activity of the ATMP, but also the quality attributes, the manufacturing process steps, and the therapeutic administration procedures. It’s important that risks be detected early and monitoring continue throughout the development of the ATMP in order to proactively manage risk and reward.
Risk identification and evaluation is essential.
Risk planning is the best way to navigate uncertainty and plan for unexpected events while being able to enhance the benefits. For example,

Once safety concerns have been pinpointed, organizations should group safety specifications into 3 categories (“important identified risks,” “important potential risks” and “missing information”) to determine how to address them. Risk minimization may require special considerations beyond routine measures. Approaches that are more ATMP-specific may include specialized trainings for qualified physicians at accredited centers and audiencefocused, targeted educational materials for treating physicians, pharmacists, patients, caregivers, family members and others.
Navigating smart clinical pathways.
Any risk assessment is typically based on pre-clinical and clinical studies data, but the standard clinical pathway may not be appropriate for ATMPs. Studies tend to be small in size and for a restricted amount of time, so very often risk assessment relies on post-authorization data since some rare side effects only appear over time. Therefore, follow-up times should be proactively considered and planned depending on the type of product.
To dispel the uncertainties that remain even after clinical studies, organizations collect safety and efficacy data obtained in “real-time” settings from post-authorization safety studies (PASS) and post-authorization efficacy studies (PAES) in the EU.
Each of these is handled on a case-by-case basis in terms of how long they last and how they’re structured. This tends to be driven by the specific characteristics of particular ATMPs, as well as the intended indication and the resulting scientific uncertainty, the important risks or missing information, as determined during the risk identification exercise.
It’s time to follow-up.
Follow-up is key since sustained, long-term clinical benefit is the promise of ATMPs and should be demonstrated as well as balanced against potential long-term risks. Therapies that involve engraftment take longer to show therapeutic effect and will need longer follow-up windows. Treatments with higher risk for tumor formation will have very stringent requirements for long-term follow-up. Assessing and addressing risk, as it relates to ATMPs is an important process. The required follow-up time typically depends on the product type, experience with related product classes as well as proper justification.
Overall, considering the benefits of these revolutionary therapies against the risk is a bit of a tightrope walk. Organizations will benefit from an experienced partner who can help navigate the risk mitigation process and bring new hope to patients who desperately need it.
Interested in learning more about how to identify and mitigate risks early in development? Download our white paper, The Future of Advanced Therapies: Strategies to Evolve Global Health in a Post-COVID World.
References:
1. Seimetz D. The key to successful drug approval: an effective regulatory strategy. J. Becker und T.R. Villinger
(Hrsg.), Life Science Venturing,
DOI 10.1007/978-3-658-06382-5_7

